
A lubricated catheter is recommended to reduce damage to the urethra and lower the risk of hematuria which is a common complication. A cross-over study comparing different hydrophilic catheters showed an even lower frequency of hematuria in patients who chose LoFric.
Intermittent catheterisation has very few contraindications, but the scientific literature recommends lubrication of the catheter to avoid trauma.1 2 3 The background to this recommendation is the complications seen after long-term use. These complications are related to damage to the urethral wall from repeated catheterisations4 5 and the fact that urethral trauma is associated with an increase in UTI risk.6 7 8 9 Damage to the urethra is more likely to occur with an un-lubricated catheter,10 and findings reported in the literature support the use of hydrophilic catheters to reduce the risk of hematuria/urethral trauma. 1 11 12 13 LoFric in particular seems to be able to reduce the risk of hematuria by 48%.14 The reported incidence of trauma varies depending on the evaluation method (e.g. self-reported bleeding, microscopic observations) and the study set-up, but the literature suggests general figures between 20-30% for patients practicing intermittent catheterisation.e.g. 15 16 17 18
LoFric documentation: Reduced risk of trauma
- Intermittent catheterisation with hydrophilic and non-hydrophilic urinary catheters: systematic literature review and meta-analyses.19
Meta-analysis concluding that the risk of UTI is reduced with the use of hydrophilic-coated catheters as compared to non-coated catheters. - Clinical and economic evaluation of LoFric® catheters for the management of bladder dysfunctions.14
Meta-analysis concluding that the risk of hematuria is 48% lower with the use of LoFric as compared to non-coated catheters. - Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials.12
Meta-analysis of 464 patients and five randomised controlled trials (RCT) with hydrophilic catheters, whereof three with LoFric. The results propose a 64% risk reduction for UTI with hydrophilic-coated compared to non-hydrophilic catheters.
This meta-analysis combines five randomised controlled trials to create stronger evidence. A meta-analysis follows strict rules on how to search, select, validate, weigh and summarise studies specified in the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) and by the Cochrane Collaboration. The studies selected for the meta-analysis were all comparing hydrophilic-coated catheters to uncoated plastic catheter in spinal cord injured patients.
The following studies were included:
- Cardenas et al. 2011: SpeediCath vs. non-coated plastic catheter in 200 newly injured patients.
- Cardenas and Hoffman 2009: LoFric vs. non-coated plastic catheters in 45 patients with injury more than 6 months ago.
- De Ridder et al. 2005: SpeediCath vs. non-coated plastic catheters in 123 newly injured patients.
- Vapnek et al 2003: LoFric vs. reused non-coated plastic catheters in 63 patients, mainly spinal cord injury.
- Sutherland et al 1996: LoFric vs. non-coated plastic catheters in 33 patients, mainly spinal cord injury.
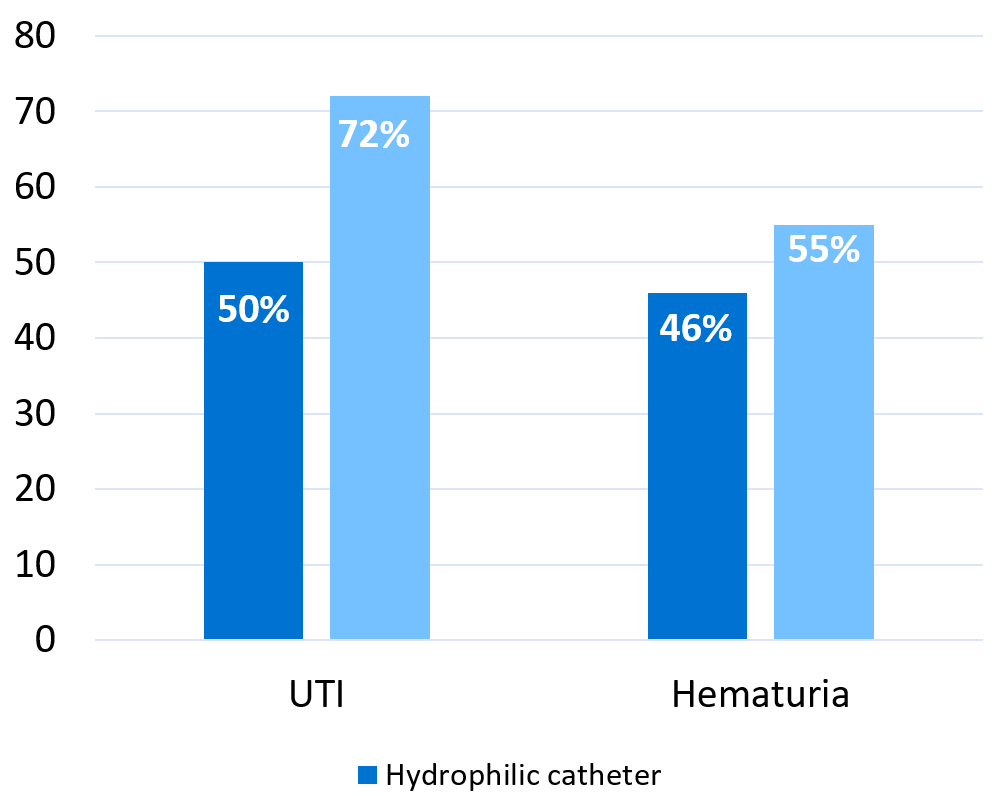
Graph based on results reported by Li et al. 201312
The publication combines the results from the studies and concludes that UTI incidence was 49.6% in patients using hydrophilic catheters and 72.0% for patients using non-hydrophilic. The results for hematuria (bleeding) were 45.7% (hydrophilic) and 55.0% (non-hydrophilic) but for this comparison only four studies could be used. The results are expressed as ‘odds ratio’ and/or ‘odds’ which is a sort of approximation for risk. The odds ratio for UTI and hematuria decreased for patients on hydrophilic catheters as follows:
- UTI: 0.36; 95% CI 24-54% (p<0.00001).
- Hematuria: 0.57; 95% CI 35-92% (p=0.001).
The figures above could be interpreted as using hydrophilic catheters, compared to non-hydrophilic ones, reduces the expected risk for UTI by 64% and hematuria by 43%.
- Hydrophilic-coated catheters for intermittent catheterisation reduce urethral micro trauma: a prospective, randomised, participant-blinded, crossover study of three different types of catheters.20
Cross-over study of LoFric and other hydrophilic/non-hydrophilic catheters in 40 healthy volunteers showing evidence of less microscopic hematuria and pain with LoFric. For example, frequency of hematuria was 40% for LoFric, 58% for SpeediCath and 67% for Incare Advance. - A prospective randomized trial of the LoFric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization.16
1 year study on 22 LoFric users and 26 users who reused non-coated plastic catheters. The results showed that the use of hydrophilic catheters are associated with less hematuria and decrease in the rate of UTI. The study suggests that patients with higher rate of UTI may benefit the most from hydrophilic catheters, and that the lower infection risk is a result of reduced urethral trauma.
This randomised controlled trial compared the use of LoFric to a non-coated plastic catheter for intermittent catheterisation. The study included 62 patients, mainly with spinal cord injury, and the follow up period was 12 months. One patient was removed from analysis due to wrongful treatment and thirteen patients were lost during the follow up. Thus, 22 patients used LoFric for 12 months and 26 used non-coated plastic catheters for 12 months. In the hydrophilic-group, patients were given 120 catheters/month (4 per day) while in the PVC-group 30 catheters were given per month (1 per day) with the instructions to clean and reuse. The results showed twice as high hematuria score among the non-coated catheter users as compared to the hydrophilic-group where no significant hematuria was seen. Decrease in UTI rate in the hydrophilic-group was seen between baseline and follow up (0.44 to 0.14 monthly per patient). In the non-coated catheter group the UTI incidence remained more or less at the same level throughout the study (0.20 to 0.14 monthly per patient). The study reports of high patient satisfaction with LoFric catheters. The publication concludes that the use of hydrophilic catheters is associated with less hematuria and a decrease in the rate of UTI as compared to non-coated catheters. It proposes that patients with higher rate of UTI may bene t the most of hydrophilic catheters, and that the lower infection risk is a result of reduced urethral trauma.
- Hydrophilic versus non-coated catheters for intermittent catheterization.21
Review of hydrophilic coated catheters including primarily LoFric studies, concluding evidence on decreased urethral irritation.
The LoFric catheter was associated with less microscopic hematuria...22
…13 of the 16 LoFric users (81%) desired to continue its use...22
- Clean intermittent catheterization in boys using the LoFric catheter.22
2-months study of 16 LoFric users and 14 users of non-coated catheters showing evidence of less hematuria with LoFric. The study reports that the LoFric group scored higher convenience and insertion comfort than the control group and that 82% wished to continue with LoFric.
...use of a LoFric catheter for intermittent catheterization was associated with significantly lesser degree of urethral inflammatory response...23
- Urethral cytology in spinal cord injury patients performing intermittent catheterisation.23
Study on 17 LoFric users, 14 users of a non-coated plastic catheter, and 11 users of an indwelling catheter. The results show evidence of LoFric causing less urethral inflammation and fewer numbers of bacteria than for those who use a non-coated plastic catheter or a urethral indwelling catheter. For example, a more than 10-fold increase in urethral inflammatory response was seen with non-coated catheters compared to LoFric. - Efficacy and safety of clean intermittent catheterization in adults.24
40 months follow up study of 41 LoFric users presenting evidence on the low incidence of urethral complications associated with LoFric. For example, no cases of urethral trauma were observed during the study period and few patients had repeated UTIs (9.8%).